Pharma & Healthcare Hi Purity Water Solutions
Water is one of the major commodities used by the pharmaceutical industry. It is used widely at raw material ingredients, solvents in the processing, formulation, and manufacture of pharmaceutical products, active pharmaceutical ingredients (APIs) and intermediates, compendial articles, and analytical reagents. Its prime uses are the reconstitution of products, during synthesis, during the production of a finished product, or as a cleaning agent for rinsing vessels, equipment, primary packing materials, etc.
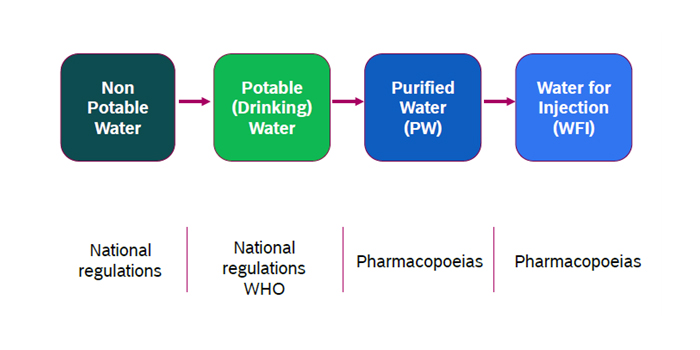
There are many different grades of water used for pharmaceutical purposes described as per USP monographs for assurance of quality. The major uses of water involve for production of bulk waters, which are typically produced on-site where they are used; and packaged waters, which are produced, packaged, and sterilized to preserve microbial quality throughout their packaged shelf life.
Sterile and high-grade treated water is desired in healthcare uses. Hospitals and healthcare facilities often use water for specific purposes and therefore have unique grades requirements from their water supplies. Whether using water for treatment, washing, and bathing, heating and cooling, or alternative applications, Tangent Engineering has been able to demonstrably produce treated water meeting desired grade for many healthcare organizations.
Water for Pharmaceutical Use
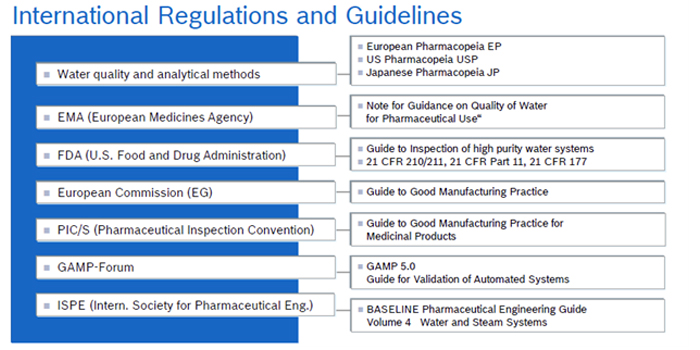
Active implementation of good quality is our priority. Pharma Water Systems are designed conforming to the requirements of FDA, cGAMP, cGMP, cUSP, & cPH Eur.
Tangent’s pharmaceutical water system products are listed belowAll systems are pre-tested, validated, compact, and ready to use. The compact, package unit design on a stainless steel skid, is specifically adapted to the high TDS water and offers all purification technologies.
- Automatic Sand and Activated carbon filters
- Duplex softening units, serially connected and quality-controlled with optional HOTwater sanitization.
- Demineralization systems
- Ultra filtration units with 6000 Daltons cutoff
- HOT water sanitizable electro-deionization units.
- UV Disinfectant
- Single or two-pass reverse osmosis
- Membrane degassing for CO2 reduction
- ORBITAL welded SS316L tubes
- State-of –the-art PLC technology.
- 21 CFR part 11 compliant control panels.
Purified Water Generation

Ultrapure Water Generation
Water for Injection Process

Purified Water Generation
Purified Water (PW) is used as diluents in the production of non-sterile products, cleaning equipment, pre-treatment in the preparation of Water for Injection (WFI), and pharmaceutical-grade pure steam production. Purified Water is also used in most of the tests and assays. Purified Water must meet the requirements for ionic and organic chemical purity and must be protected from any microbial contamination.
The treatment process for the generation process includes deionization, distillation, ion exchange, reverse osmosis, filtration, or other suitable purification procedures. Purified water systems must be validated to reliably and consistently produce and distribute water of acceptable chemical and microbiological quality.
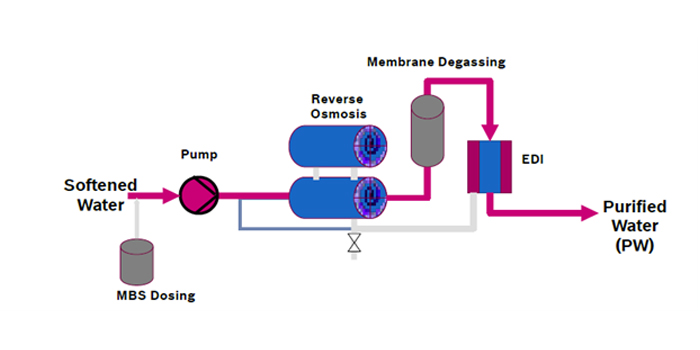

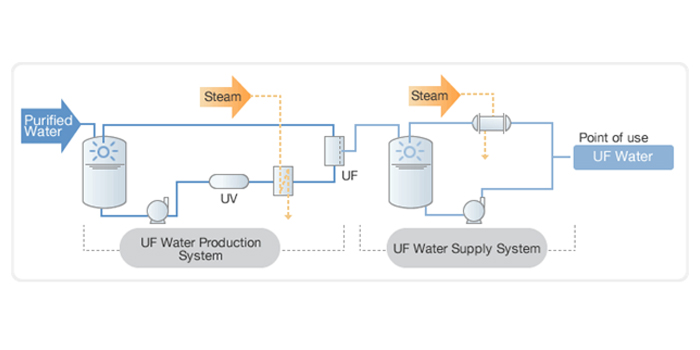
Water for Injection ( WFI)
Pharmaceutical and biotechnology industries utilize Sterile Water for Injection (WFI) as per pharmaceutical standard norms. This WFI is produced by the highest grade water treatment systems. WFI is utilized in injectable drugs, hemofiltration, for producing active pharmaceutical ingredients, implantable medical devices, and other varied applications. .
WFI is of higher quality than purified water and has its standards set out by the worldwide pharmacopoeias (European Pharmacopoeia, United States Pharmacopoeia, Japanese Pharmacopoeia, etc.).
.Water for Injection (WFI) is generated through distillation and further purification by Reversed Osmosis.Water for Injection systems must be validated to reliably and consistently produce and distribute this quality of water.
Water for Injection systems must be validated to reliably and consistently produce and distribute this quality of water.

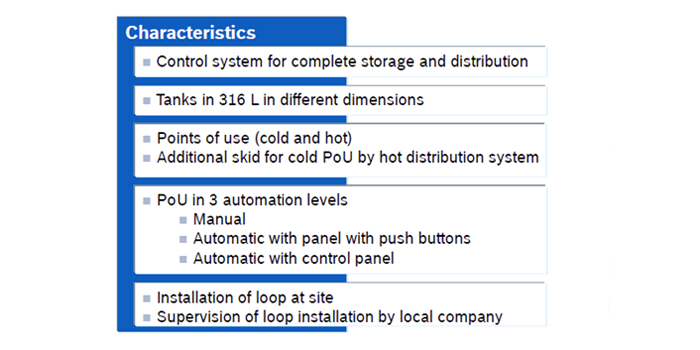
Storage and Distribution Loop
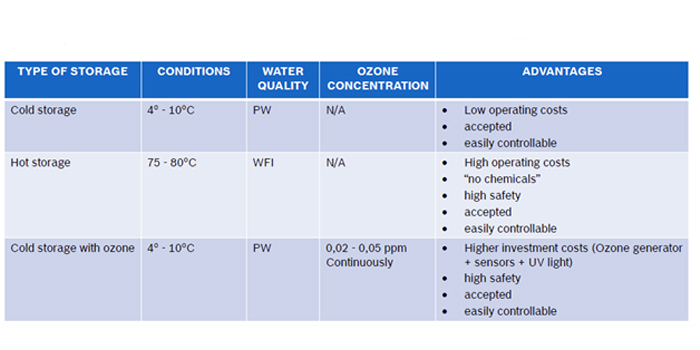
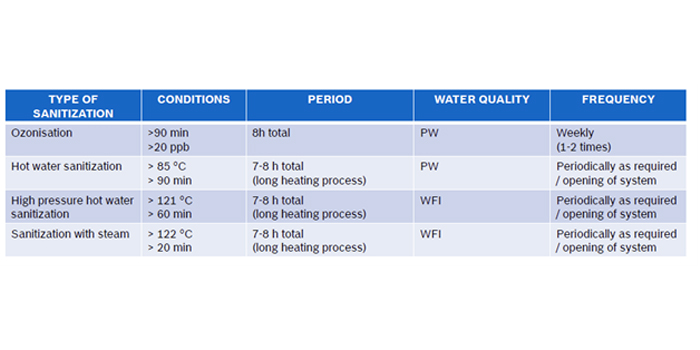
The pharmaceutical water distribution system is typically structured in a closed circulation loop, assuring turbulent water motion in the pipes 24 hours a day and 7 days a week. Purified Water loop, most often works in a cold mode, at a temperature in the range of 15 - 25 °C. WFI water is usually stored hot, at a temperature > 80 °C.
The main components of the water distribution system are a storage tank and a circulation pump or pumps. Storage tanks are used for managing peaks in water use. Circulation pumps are responsible for ensuring turbulent flow in pipelines and maintain the required pressure in the system. Depending on the number and location of user points, the distribution system may contain one or more circulation loops starting and ending in the storage tank.
Very often, UV units for water disinfection devices are installed at the PW loop feed lines to minimize the risk of bacterial growth in the system. UV disinfection devices which are installed in water distribution systems for the pharmaceutical industry must be free from dead legs and completely drainable.



Hospital and Healthcare Water Treatment
Water, Sanitation, and Hygiene programmed from WHO has standardized guidelines for water quality, quantity, and access required in healthcare facilities. Typical drinking water can contain a range of microorganisms, including Pseudomonas aeruginosa, non-tuberculous Mycobacterium spp., Acinetobacter spp., Aeromonas spp. and Aspergillus. Etc. hence additional processing may be required to ensure safety for consumption by severely immune-suppressed persons, such as those with neutrophil counts below 500 per ml. Microorganisms in drinking water also have the potential to cause infections if drinking water is used to wash burns or to wash medical devices such as endoscopes and catheters. Water used for such purposes needs to be of a higher quality than described in these Guidelines of the drinking water and may require additional processing, such as microfiltration or sterilization, depending on use.
- Water treatment for potable and general uses
- Water treatment for dialysis use
- Water treatment for sterile use.
- Ultraviolet TOC Reduction
- Ultraviolet Disinfection Systems
- Reverse Osmosis
- Ultrafiltration
- Deionized Water Systems
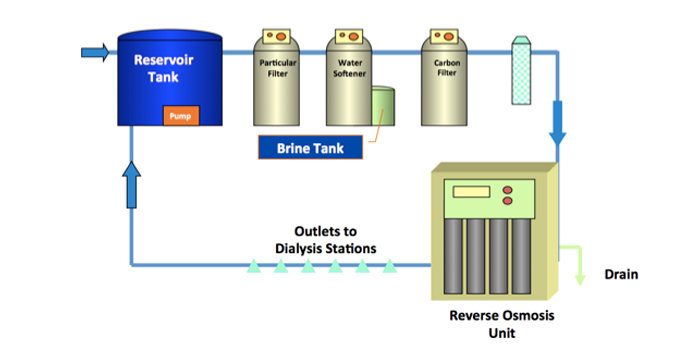
Water for Hemodialysis
During an average week of hemodialysis, each patient can be exposed to 300-600 liters of water, providing multiple opportunities for potential patient exposure to waterborne pathogens. Adverse patient outcomes including outbreaks associated with water exposure in dialysis settings have resulted from patient exposure to water via a variety of pathways; including improper formulation of dialysate with water containing high levels of chemical or biological contaminants, contamination of injectable medications with tap water, and reprocessing of dialyzers with contaminated water. For the health and safety of hemodialysis patients, it is vital to ensure the water used to perform dialysis is safe and clean.